Improve Clinical Trial Efficiencies
and Save Staff Time
5thPort helps accelerate trials and improve study success by combining digital participant engagement and eConsent – in one centralized hub. We create effortless participant and staff experiences, giving time back to your staff and ultimately, maximizing efficiency.
Supports:
- Sponsors
- Contract Research Organization
- Research Institutions
- Research Sites and Site Networks
Learn how 5thPort can ease your clinical research team’s workload.
Strive For Seamless Trial Operations
With Centralized Study Management Capabilities
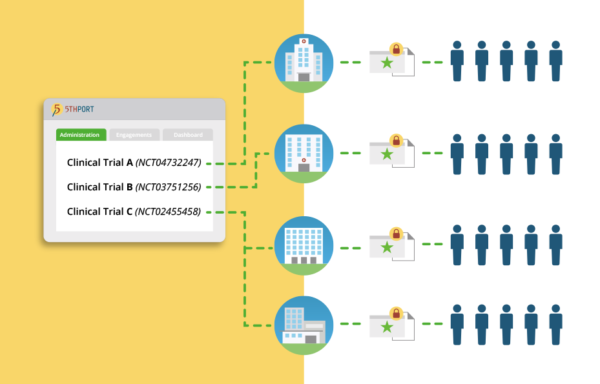
In trials that span boundaries and jurisdictions, 5thPort enables the creation and the digital distribution of standardized study materials and consent forms to multiple sites simultaneously.
Get real-time monitoring of all consent activities within a single dashboard. Review and analyze enrollment results across all participating sites and researchers.
Centrally manage all consent and engagement artifacts. Version control and automated re-consenting ensures every participant is signing the right version of the consent every single time.
Speak with us to understand how we can enhance efficiencies while achieving cost reductions.
Improve Quality of Participant-Clinician
Conversations. Reduce Attrition Rates.
Free up clinician time that could be used for more insightful and valuable discussions during site visits.
5thPort helps empower your staff to engage participants at their convenience and foster a higher level of motivation by “pre-educating” them prior to the start of a trial. Enable participants to ask questions and flag consent sections for clarification digitally, thereby reducing the amount of time you spend explaining the trial protocol.
Let’s discuss how 5thPort can ease your time constraints and your research team’s workload.
