5thPort for Clinical Trials: Participant-Centric Education and eConsent Software
Clinical trials thrive on participant trust, understanding, and engagement, yet many trials falter due to low retention, compliance challenges, and poor patient comprehension. 5thPort transforms these challenges into opportunities with a platform that seamlessly integrates cutting-edge patient education and eConsent software capabilities, empowering participants while streamlining trial operations.
From rich educational videos powered by AI avatars to intuitive electronic consent workflows, 5thPort equips researchers, sponsors, and sites with everything they need to deliver truly informed consent. Participants aren’t just signing forms; they’re gaining clarity, building confidence, and fostering trust in the trial process.
Whether managing hybrid, virtual, or decentralized clinical trials, 5thPort’s platform ensure you hit your DE&I requirements (learn more) in a compliant environment (learn more). By simplifying complex protocols into accessible formats and providing multilingual support, we enhance participant understanding and satisfaction across diverse populations. With 5thPort, clinical trials are not just easier to run – they’re smarter, faster, and more participant-centered.
Clinical Trial Testimonial
“Eurofins is a global leader in food, environmental, pharmaceutical and cosmetic product testing services. With multiple locations, we were looking to streamline and simplify our quality assurance processes. After performing due diligence on other solutions in the marketplace, we decided to move forward with 5thPort to implement their cutting-edge panelist engagement and informed consent solution. Our experience with both the solution and the company has been great.
I highly recommend 5thPort to other organizations in the clinical trial space.”

Lenore Coyle, President, Eurofins CRL, Cosmetics, Inc.
Enhance Participant Education
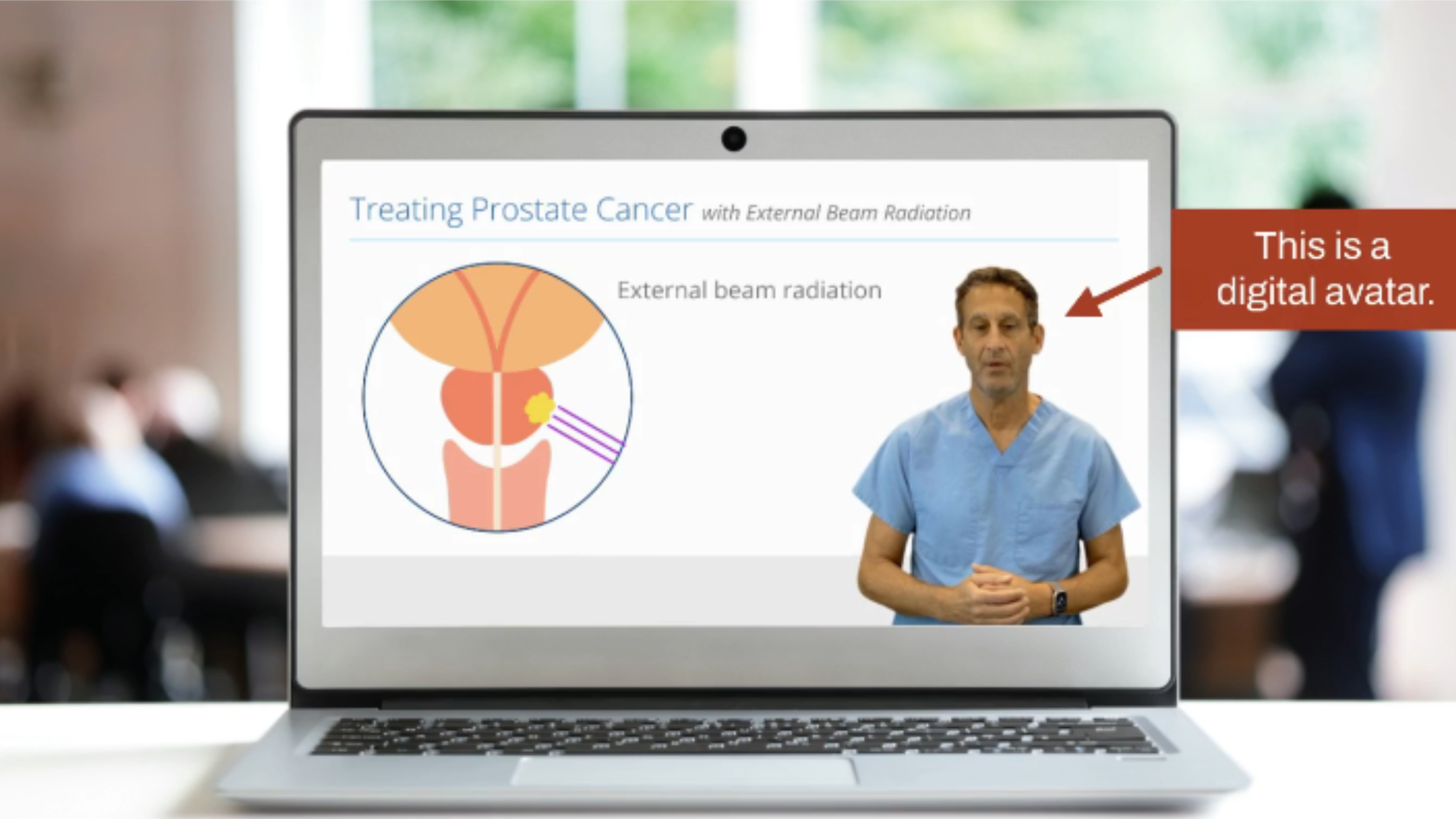
Provide tailored, multimedia-rich educational content for participants using 5thPort’s AI-powered digital avatars.
Ensures comprehension through in-built interactive quizzes and surveys that inculcate the teach-back method.
Break down complex medical information into plain language text, documents, videos and visuals in a combination your patient is most comfortable with.
Build participant confidence and trust in the trial process.

Ensure Regulatory Compliance
Meet regulatory standards with audit-ready documentation through our proprietary One Touch Compliance Report™ which identifies who has performed which step, in real-time.
Simplify version control for decentralized, hybrid, and multi-site trials by ensuring participants always interact with the latest version of engagement content – including consent forms.
5thPort is externally validated and in compliance with 21 CFR Part 11, EU Annex 11, ICH-GCP (electronic records and signatures) and HIPAA (security and encryption).
Eliminate expensive informed consent related audit findings.
Streamline the Consent Process with eConsent Software
Offer digital, interactive eConsent forms for easier understanding and engagement. Build your own forms from scratch, or speak with us for one you can use.
Include secure digital signatures in a HIPAA and 21 CFR Part 11 compliant environment with our eConsent software.
Track participant progress in real-time with our proprietary One Touch Compliance Report™.
Facilitate better access to educational material, enabling participants to choose to receive communication about their engagement plan via SMS and/or email.
Automate reconsenting by ensuring participants only sign the most up-to-date versions of forms.


Drive Participant Retention
Support participant adherence with personalized engagement tools – AI-powered avatar videos, documents, quizzes and surveys – customized to a participant’s treatment options, disease site, diagnosis and more.
Reduce dropout rates by limiting the amount of on-site and follow-up visits required, especially those that can be completed remotely.
Analyze which engagement assets (videos, documents and more) are being understood by trial participants and which aren’t, and make those changes/substitutes in real-time.
Provide on-demand access to educational materials for continuous engagement.
Promote Diversity, Inclusion and Equity (DE&I)
Eliminate cultural barriers to participation with inclusive browser-based and device-agnostic technology, and educational assets.
Support multilingual education and consent to accommodate global participant populations. 5thPort supports 80+ languages, including those from R-L.
Build participant trust in the trial process by enabling them to complete the engagement process remotely from the comfort of their own home, at their own pace.
Ensure participants from diverse backgrounds feel valued and supported with accessible and culturally relevant videos, documents, surveys and quizzes.

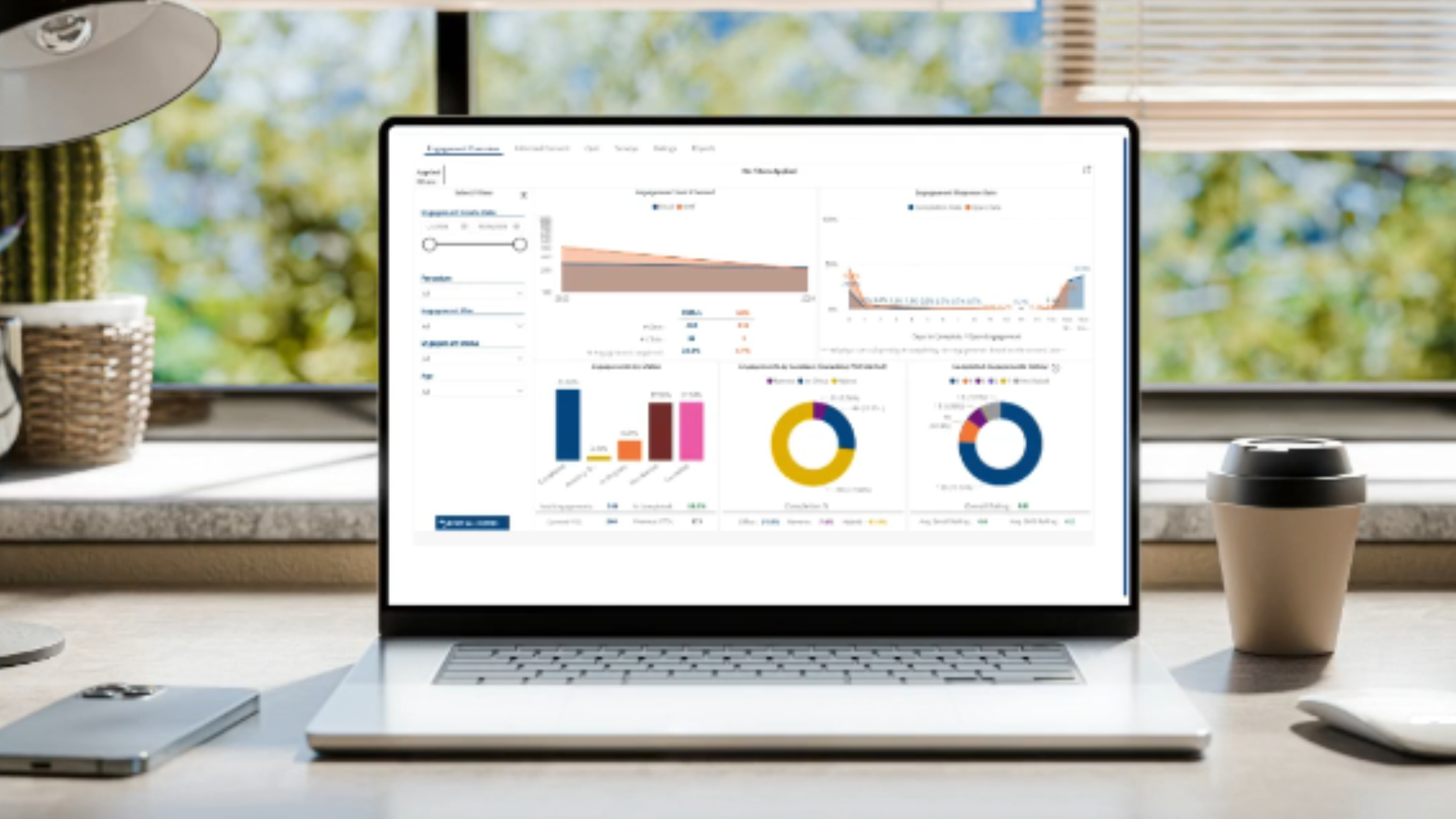
Deliver Real-Time Actionable Insights
Provide real-time analytics on participant comprehension and engagement levels.
Identify areas for improvement in education materials and processes.
Help trial administrators refine strategies for better outcomes.
Track minority accrual metrics overtime to aid in trial generalizability.