Best-in-class eConsent software: improve trial retention rates, protocol adherence.
When clinical trial participants better understand trial protocols and associated risks and benefits, they are able to have a meaningful consent conversation where their questions and concerns are addressed. This digital clinical trial engagement favorably impacts participant conversion, protocol adherence, and retention rates.
5thPort’s eConsent software is digital, simplifying clinical trials consent related audit and regulatory compliance. It also helps to drive Health Equity in decentralized clinical trials.
5thPort’s eConsent software delivers more than just better health outcomes…
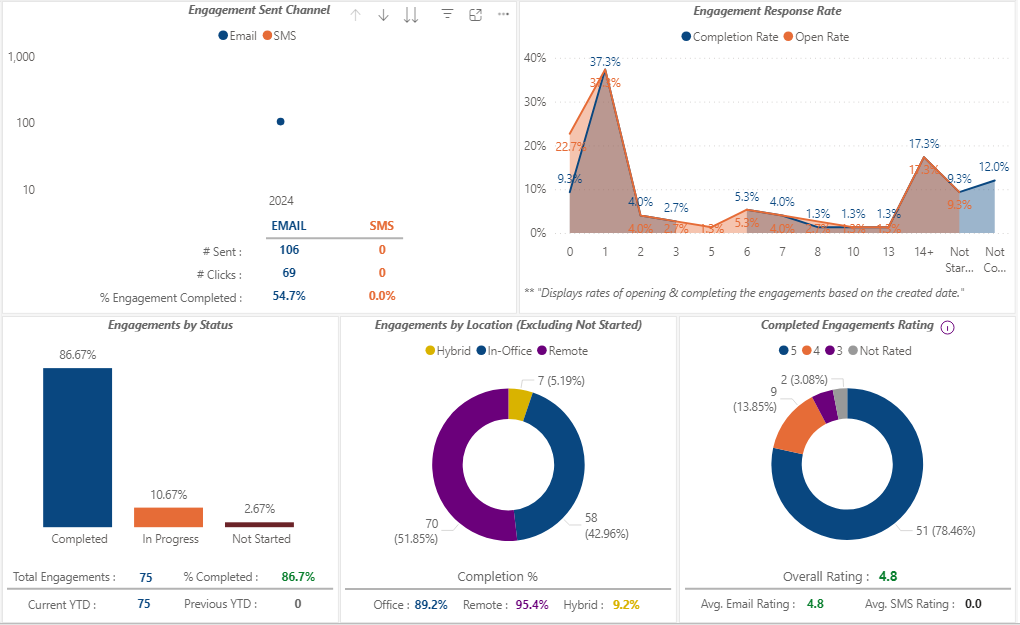
Our eConsent software brings you real-time analytical prowess you can trust.
Allow our analytics to guide your assessment of patient education and eConsent effectiveness and identify opportunities to improve your patient’s comprehension of medical information presented to them.
With 5thPort’s eConsent software, you can analyze response trends across engagement channels (email and SMS), understand the effectiveness of your videos, questionnaires, surveys, and teach-back. Armed with this data, you have the opportunity to adjust your workflows to gauge and improve clinical trial participant satisfaction in near real-time.
In an era of increasing compliance requirements, 5thPort’s eConsent software reduces the risk of regulatory penalties.
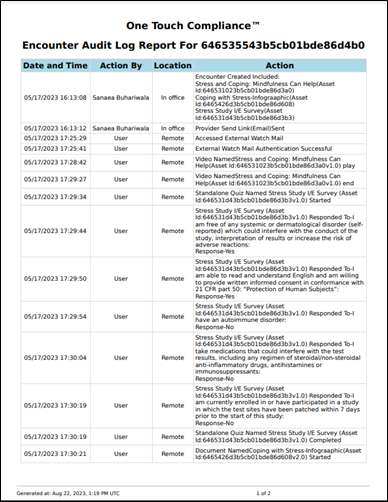
The convenient One Touch Compliance (OTC) Report digitally documents every action taken by clinical trial participants and staff during the patient education and eConsenting process – including time and date stamps, as well as, the staff who completed said action. 5thPort’s eConsent software also ensures seamless version control and efficient re-consenting, so patients always receive the most up-to-date information while maintaining full regulatory compliance.
What does that mean for you?
1. Digital documentation at your fingertips
2. No stress compliance with internal or external consent audit requests
3. Solid risk mitigation