Electronic Informed Consent Software by 5thPort
eConsent for Clinical Research Organizations (CROs) and Sponsors
Successful Clinical Trials demand simple, standard, and effective Electronic Informed Consent Software.
As a digital patient engagement and eConsent platform provider, 5thPort enables sponsors and clinical research organizations (CRO’s) to leverage multi-media content, comprehension testing techniques and a host of automated trial management capabilities to:
- Better operational efficiencies with ICF in clinical research – Informed Consent Forms
- Lower operational expenses
- Eliminate staff’s stress related to consents and audits
- Educate while empowering patients
- Drive more meaningful patient conversations
Our comprehensive eConsent and electronic informed consent software keeps track of complicated patient engagement and informed consent workflows, including re-consenting, so you don’t have to.
Unleash the power of true patient comprehension and electronic informed consent software. Get in touch.
Multi-site and multi-jurisdiction ready
Patient engagement assets in 5thPort can be customized by either site or jurisdiction while still allowing the study to be managed centrally by the sponsor or Clinical Research Organization (CRO).
This ensures the most up-to-date and accurate content assets are being used by all sites/jurisdictions.
Meet your eConsent compliance protocol management ally
We’ve designed a compliance protocol for your consenting process that outshines the rest.
- Your QA and compliance teams can validate each patient’s eConsent record(s) and/or download them for archiving purposes.
- Patients can flag sections that they have questions about and digitally document them for follow up. No more scribbling on paper!
- eConsent cannot be taken until all the patient’s questions have been answered.
Manage Informed eConsent Form assets from one central location. Unlock the power of electronic ICF in Clinical Research.
Do you struggle to manage the various versions of informed consent forms and other consent-related assets?
With 5thPort’s eConsent form version control capabilities – sponsors and CRO’s can centrally manage asset versions.
Once the latest approved version is published, our electronic informed consent software solution automatically pushes that out to all participating sites – essentially superseding all older versions in real-time. This is the power of ICF in clinical research.

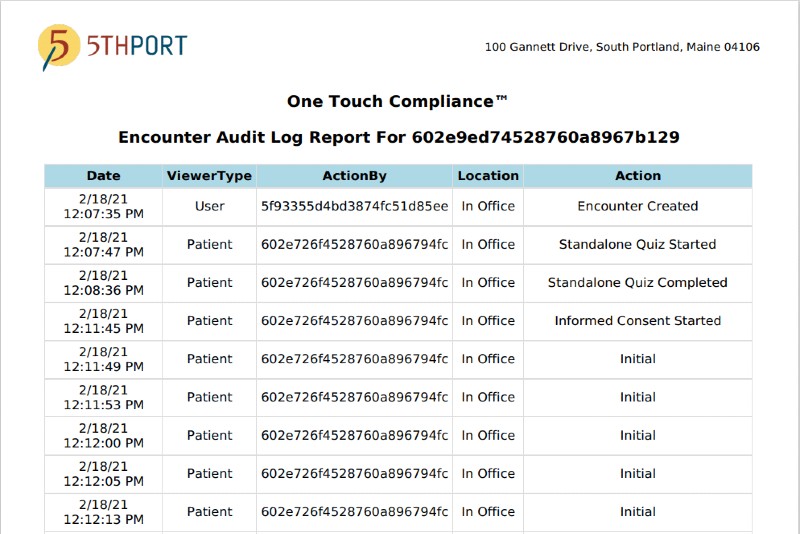
5thPort’s Electronic Informed Consent Software One Touch Compliance Report™ equals for solid risk mitigation.
Digitally document every action taken in 5thPort’s electronic informed consent software during every patient’s consenting process.
This means a no-stress compliance workflow with internal or external eConsent audit requests.
5thPort’s One Touch Compliance Report records every encounter action in a one of kind log report leading to a stress-free audit.
Easy to use, easy to implement eConsent for both staff and patients | eConsent Form
We recognize the importance of getting new therapies to market quickly and efficiently. A true SaaS platform, 5thPort electronic informed consent software can be set up in as little as 2-3 weeks depending on the size of your study.
Adopting 5thPort for your patient engagement and informed consent needs is intuitive – for your staff and your patients.
We guarantee a dedicated training and onboarding manager to your account. eConsent implementation and training are structured and can be customized to your specific needs.
