Retain participants and improve compliance at a lower cost
5thPort helps accelerate trials and improve study success by combining digital engagement and eConsent – all in one place. We create effortless participant and staff experiences, simplify trial operations, and maximize workforce efficiency.
Supports
- Sponsors
- Contract Research Organizations
- Research Institutions
- Research Sites and Site Networks

Reduce compliance risk
No more incorrect, incomplete, altered
or lost consent forms.
5thPort captures every action on the platform with a digital date/time stamp for bullet proof documentation. The platform ensures that participants always interact with the latest version of engagement content including consent forms.
The completed consent forms are available digitally (PDFs) for download at any time during your trial, and all consent forms for the trial can be downloaded for your records when the trial is complete.
5thPort is externally validated and in compliance with 21 CFR Part 11, EU Annex 11, ICH-GCP (electronic records and signatures) and HIPAA (security and encryption).
With the click of a button, our One Touch Compliance™ report gives researchers the detailed information required for audit processes.



Increase trial efficiency
Save staff time.
The digital engagement protocol will eliminate repetitive conversations with each of your consenting participants and deliver consistent standardized messaging.
To facilitate better access to educational material, users can choose to receive communication about their engagement plan via SMS and/or email. Re-consenting is a simple and completely automated process.
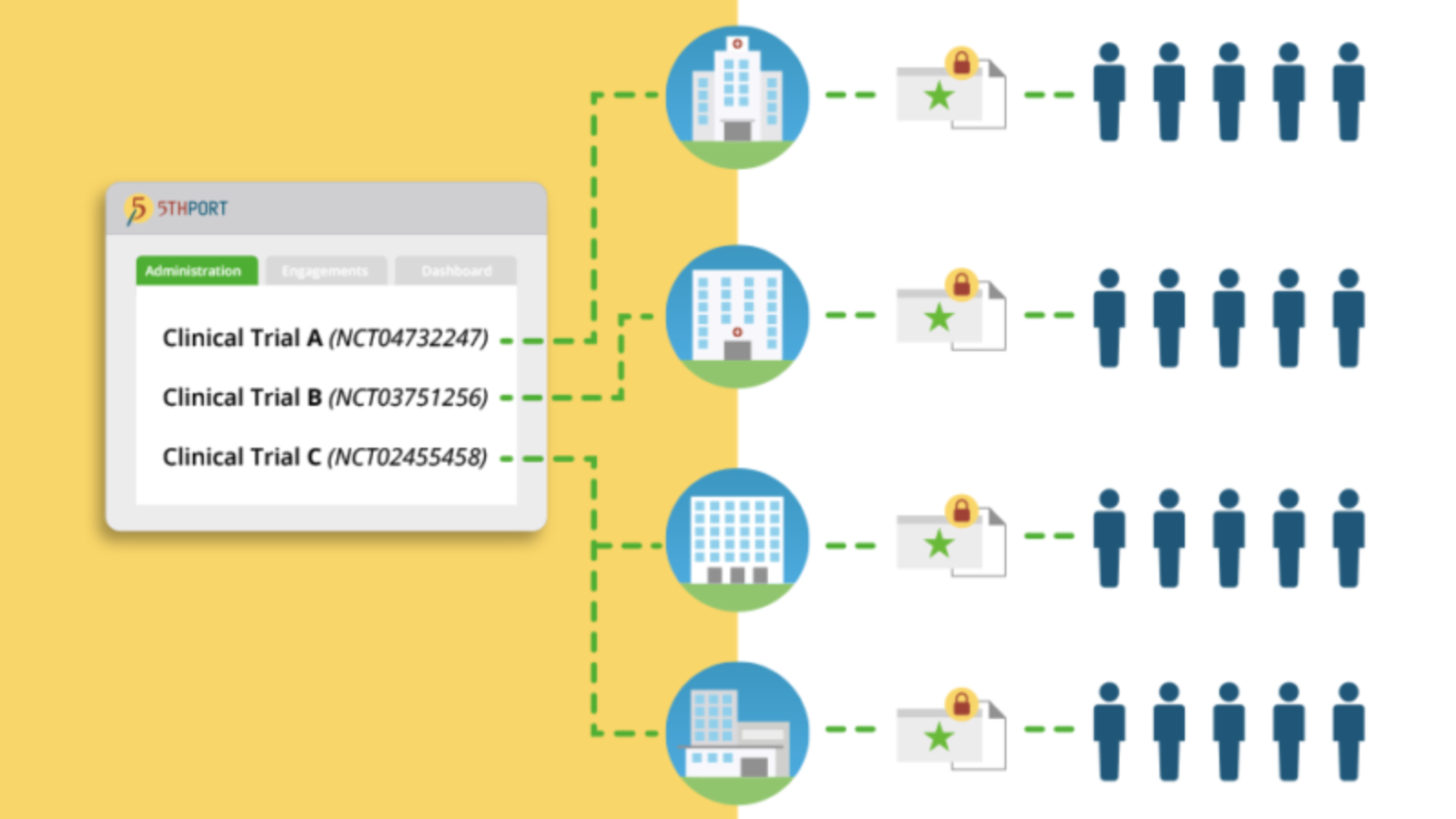
Better manage clinical trials
Gain visibility and oversight with centralized study management capabilities.
Get real-time monitoring of all consent activities within a single dashboard. Review and analyze enrollment results across all participating sites and researchers.
Centrally manage all consent and engagement artifacts. Version control and automated re-consenting ensures every participant is signing the right version of the consent every single time.

Inspection audit ready
Stress-free compliance with automated processes.
Let’s face it, keeping track of paper consent forms is no fun, especially when faced with an audit. 5thPort captures every action on the platform with a digital date/time stamp for bullet proof documentation.
Quality role specific functionality lets QA personnel review and audit consent and re-consent forms for stress free compliance.
The completed consent forms are available digitally (PDFs) for download at any time during your trial, and all consent forms for the trial can be downloaded for your records when the trial is complete. Reconsenting is a simple and completely automated process.
