5thPort for Clinical Trials: Participant-Centric Education and eConsent Software
Clinical trials thrive on participant trust, understanding, and engagement, yet many trials falter due to low retention, compliance challenges, and poor patient comprehension. 5thPort transforms these challenges into opportunities with a platform that seamlessly integrates cutting-edge patient education and eConsent software capabilities, empowering participants while streamlining trial operations.
From rich educational videos powered by AI avatars to intuitive electronic consent workflows, 5thPort equips researchers, sponsors, and sites with everything they need to deliver truly informed consent. Participants aren’t just signing forms; they’re gaining clarity, building confidence, and fostering trust in the trial process.
Whether managing hybrid, virtual, or decentralized clinical trials, 5thPort’s platform ensure you hit your DE&I requirements (learn more) in a compliant environment (learn more). By simplifying complex protocols into accessible formats and providing multilingual support, we enhance participant understanding and satisfaction across diverse populations. With 5thPort, clinical trials are not just easier to run – they’re smarter, faster, and more participant-centered.
Clinical Trial Testimonial
“Eurofins is a global leader in food, environmental, pharmaceutical and cosmetic product testing services. With multiple locations, we were looking to streamline and simplify our quality assurance processes. After performing due diligence on other solutions in the marketplace, we decided to move forward with 5thPort to implement their cutting-edge panelist engagement and informed consent solution. Our experience with both the solution and the company has been great.
I highly recommend 5thPort to other organizations in the clinical trial space.”

Lenore Coyle, President, Eurofins CRL, Cosmetics, Inc.
Enhance Participant Education
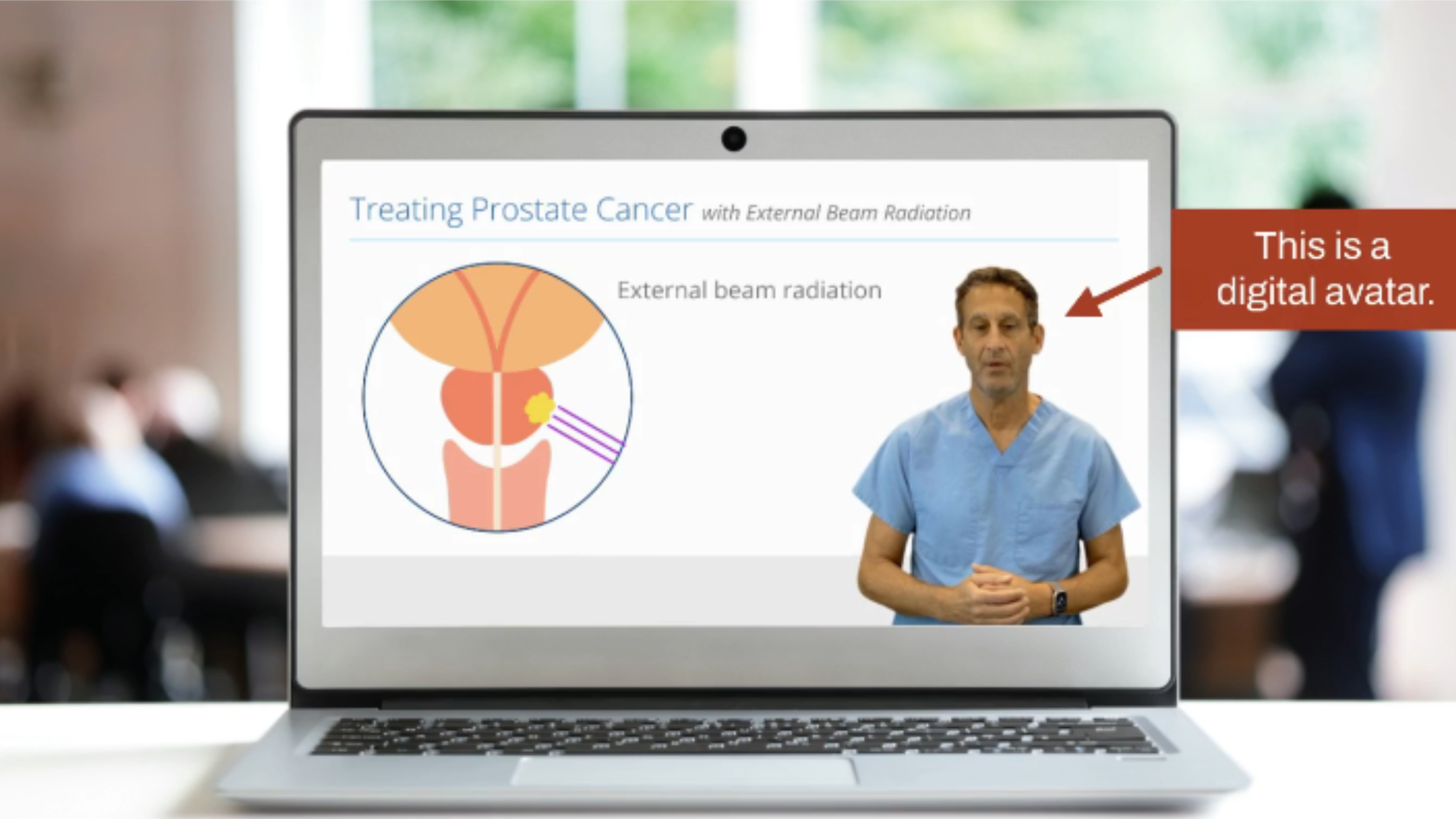
Streamline the Consent Process with eConsent Software


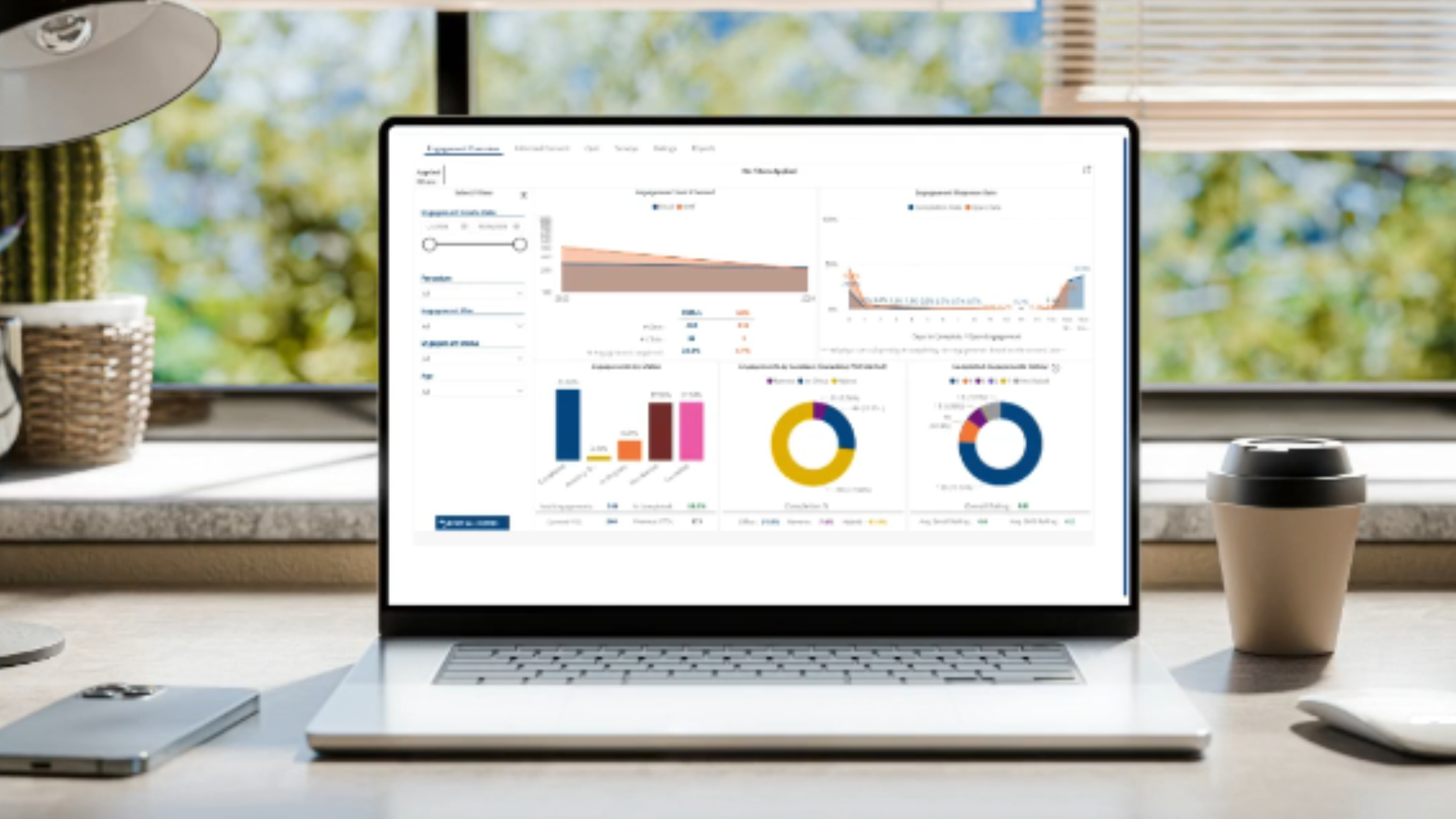
Drive Participant Retention