Drive DEI (Diversity, equity and inclusion)
with 5thPort patient engagement & eConsent
Diverse representation in clinical trials is critical. Lifesaving medicines for all begins with doing research for all. Adherence to the FDA guidance on more participants from underrepresented populations in clinical trials is being enforced. Are you prepared?
5thPort can help you meet your diversity goals by providing access to participants anywhere, any time, on any device, delivering multi-media content for more meaningful participant understanding.
- Engage participants in their language of choice
- Tailor outreach for cultural sensitivity
- Teach-back to reinforce understanding
Learn more about how 5thPort can drive DEI in your clinical trials.
Ensure scientific validity and generalizability.
Cater to a broader patient demographic and deliver applicable trial results.
Pre-trial information arms patients with essential trial knowledge, enabling informed consent and more accurate outcomes. Two-way communication fosters patient input, while remote accessibility features address geographical and mobility barriers.


Make participants feel safe and secure
during their clinical trial.
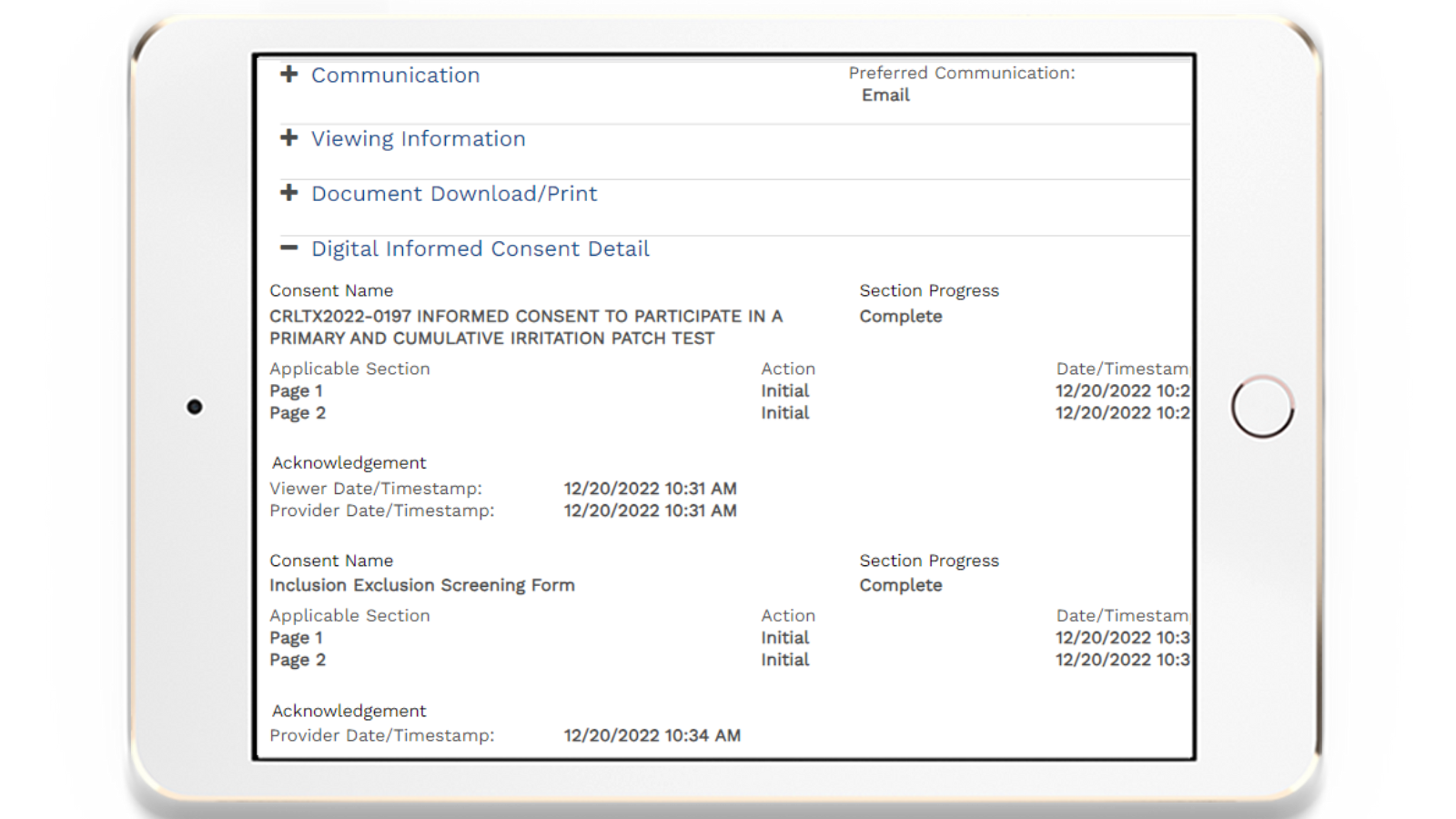
An engaged participant is a committed participant – which creates better clinical outcomes. 5thPort can help participants better understand trial requirements and make informed decisions – fostering trust and confidence.
Speak with us about your organization’s DEI goals and learn how we can help you empower underrepresented communities in your next clinical trial.
RETAIN TRIAL PARTICIPANTS
DID YOU KNOW: 40% of participants become “non-compliant” 150 days into a clinical trial?